TPSAC unconvinced
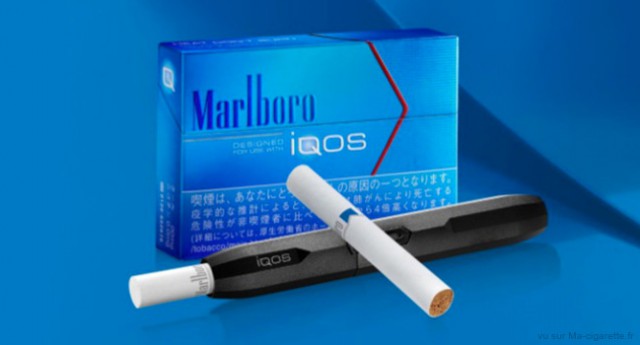
The Tobacco Products Scientific Advisory Committee (TPSAC) said evidence does not support Philip Morris International’s claim that its heat-not-burn product, iQOS, reduces the risk of tobacco-related diseases.
The panel also rejected a claim that iQOS is less risky than continuing to smoke cigarettes.
The committee voted in favor of the claim that iQOS reduces the body’s exposure to harmful or potentially harmful chemicals but against another that reductions in exposure are reasonably likely to translate to a measurable and substantial reduction in morbidity and or mortality.
Panelists said they think the likelihood iQOS users switch completely to the device is low to medium.
The Food and Drug Administration is not required to follow TPSAC’s recommendations, though historically the regulatory agency has followed the committee’s advice.
Despite today’s setback, Wells Fargo analyst Bonnie Herzog believes that the chances for the FDA to ultimately approve PM’s MRTP are still good, although the timing is tough to predict.
“We don’t view today’s events as a total disaster given [that] much of the panel’s concerns were around how PM worded its proposed reduced-risk claims and how PM ‘messages’ those claims to the consumer,” she wrote.
Herzog took comfort from the fact that the TPSAC’s recommendation is non-binding, and that PM’s MRTP application is in line with the FDA’S plan for a comprehensive nicotine strategy.
PMI has another application under FDA review that would simply allow iQOS to be sold in the U.S., without the lower-risk claims.